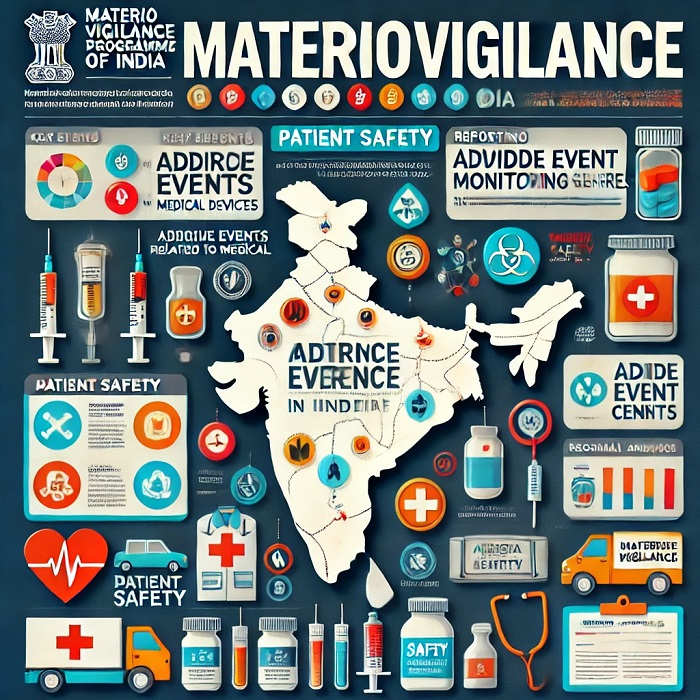
The Role of Materiovigilance in Ensuring Patient Safety in India
The healthcare industry heavily depends on medical devices for diagnosing, monitoring, and treating patients. These devices play a vital role in saving lives, their malfunction or improper use can result in adverse events, posing significant risks to patient safety. This is where Materiovigilance, a system of monitoring medical devices, comes into the picture. In India, the Materiovigilance Programme of India (MvPI) is pivotal in ensuring that the use of medical devices remains safe and effective. Let’s get into how Materiovigilance plays a role in enhancing patient safety in the country.

What is Materiovigilance?
Materiovigilance is the systematic monitoring of medical devices to detect, assess, report, and prevent adverse events. It involves tracking device performance and safety post-market, ensuring that any risks associated with their use are identified and mitigated in a timely manner. The main goal is to minimize harm to patients by identifying defective devices and preventing their continued use.
In India, the Materiovigilance Programme of India (MvPI) was launched in 2015 under the Central Drugs Standard Control Organization (CDSCO) and Indian Pharmacopoeia Commission (IPC). The initiative is part of a broader global effort to enhance patient safety through vigilant monitoring of medical devices.
Importance of Materiovigilance in Patient Safety
- Early Detection of Adverse Events:- Materiovigilance enables healthcare providers to report issues with medical devices as soon as they occur. Early detection of malfunctioning or harmful devices ensures timely action, preventing further adverse events. For instance, if a device shows signs of wear or produces inaccurate readings, early reporting can lead to its removal from use, protecting patients from potential harm.
- Improving the Quality of Medical Devices:- By systematically collecting data on device-related adverse events, Materiovigilance helps manufacturers identify recurring problems. This feedback loop encourages manufacturers to improve the design and safety of their devices. It also helps regulatory authorities ensure that only high-quality devices are allowed in the market, directly contributing to better patient outcomes.
- Strengthening Regulatory Oversight:-Materiovigilance is integral to regulatory decision-making. By analyzing adverse event data, regulatory bodies like CDSCO can make informed decisions on whether to recall faulty devices, issue safety alerts, or mandate design improvements. This regulatory oversight helps ensure that medical devices on the market are safe and reliable.
- Enhancing Public Health:-Unsafe medical devices can result in widespread public health concerns, especially in critical areas like diagnostics, surgery, and intensive care. The timely reporting and management of device-related issues through Materiovigilance prevent widespread adverse effects, ensuring that public health remains safeguarded.
- Empowering Healthcare Providers and Patients:-Materiovigilance empowers healthcare providers and patients by promoting a culture of vigilance and safety. Healthcare professionals are encouraged to report any device malfunctions or adverse events, fostering a proactive approach to patient safety. This system also helps build trust among patients, knowing that the devices used in their treatment are continuously monitored for safety.
How the Materiovigilance Program Operates in India
The Materiovigilance Programme of India operates through a well-structured network:
- National Coordination Centre (NCC): The Indian Pharmacopoeia Commission (IPC) acts as the NCC for MvPI, overseeing its operations and providing technical guidance.
- Central Drugs Standard Control Organization (CDSCO): The CDSCO serves as the central regulatory body, ensuring that all adverse event reports are thoroughly evaluated and acted upon.
- Adverse Event Monitoring Centres (AMCs): Various healthcare institutions across India act as AMCs, responsible for collecting and reporting data on device-related adverse events. These centers play a crucial role in gathering real-world data on device performance.
- Technical Support Unit (TSU): Based at the National Health Systems Resource Centre (NHSRC), the TSU provides operational support to AMCs and helps maintain the integrity of the data collected.
Challenges Facing Materiovigilance in India
Despite its significance, the Materiovigilance Program faces several challenges:
- Lack of Awareness: Many healthcare professionals are unaware of the Materiovigilance system or its importance. This leads to under reporting of adverse events, limiting the program’s effectiveness.
- Insufficient Resources: Some healthcare institutions may lack the resources or infrastructure to properly monitor and report device-related adverse events.
- Complex Medical Device Market: With a vast array of medical devices on the market, ensuring the effective monitoring of all devices is a daunting task. The diverse range of devices, from simple syringes to complex imaging machines, makes standardization difficult.
Future of Materiovigilance in India
The future of Materiovigilance in India looks promising, with the growing importance of medical devices in healthcare. To improve its reach and impact, there needs to be:
- Greater Awareness and Training: More healthcare professionals need to be trained on the importance of reporting adverse events. Initiatives that promote awareness and education around Materiovigilance will boost participation in the program.
- Enhanced Infrastructure: Upgrading healthcare infrastructure, particularly in smaller hospitals and clinics, will improve the reporting and monitoring of adverse events.
- Technology Integration: Leveraging digital platforms for real-time reporting and data analysis will make Materiovigilance more efficient, allowing for faster response times in managing device-related issues.
Conclusion
Materiovigilance plays a critical role in ensuring patient safety by monitoring and managing the risks associated with medical devices. The Materiovigilance Programme of India (MvPI) provides a structured framework for identifying and addressing issues with medical devices, ultimately enhancing the quality of healthcare across the country. With increased awareness, regulatory support, and improved infrastructure, Materiovigilance can significantly contribute to a safer healthcare environment in India.







Leave a Reply